Reinventing depression treatment via transcranial magnetic brain stimulation (TMS)
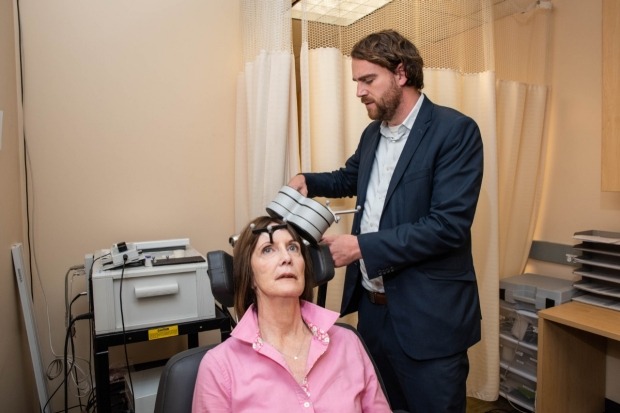
Deirdre Lehman with Nolan Williams, who oversaw this clinical trial of a potential treatment that uses transcranial magnetic stimulation. In the photo, Williams and Lehman demonstrate how a patient is positioned and the equipment is used. Credit: Steve Fisch.
___
Stanford researchers devise treatment that relieved depression in 90% of participants in small study (press release):
“A new form of magnetic brain stimulation rapidly relieved symptoms of severe depression in 90% of participants in a small study conducted by researchers at the Stanford University School of Medicine … In transcranial magnetic stimulation, electric currents from a magnetic coil placed on the scalp excite a region of the brain implicated in depression. The treatment, as approved by the FDA, requires six weeks of once-daily sessions. Only about half of patients who undergo this treatment improve, and only about a third experience remission from depression.
Stanford researchers hypothesized that some modifications to transcranial magnetic stimulation could improve its effectiveness. Studies had suggested that a stronger dose, of 1,800 pulses per session instead of 600, would be more effective. The researchers were cautiously optimistic of the safety of the treatment, as that dose of stimulation had been used without harm in other forms of brain stimulation for neurological disorders, such as Parkinson’s disease.
Other studies suggested that accelerating the treatment would help relieve patients’ depression more rapidly. With SAINT, study participants underwent 10 sessions per day of 10-minute treatments, with 50-minute breaks in between.”
About the Study:
Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression (American Journal of Psychiatry). From the abstract:
- Objective: New antidepressant treatments are needed that are effective, rapid acting, safe, and tolerable. Intermittent theta-burst stimulation (iTBS) is a noninvasive brain stimulation treatment that has been approved by the U.S. Food and Drug Administration for treatment-resistant depression. Recent methodological advances suggest that the current iTBS protocol might be improved through 1) treating patients with multiple sessions per day at optimally spaced intervals, 2) applying a higher overall pulse dose of stimulation, and 3) precision targeting of the left dorsolateral prefrontal cortex (DLPFC) to subgenual anterior cingulate cortex (sgACC) circuit…
- Results: One participant withdrew, leaving a sample size of 21. Nineteen of 21 participants (90.5%) met remission criteria (defined as a score <11 on the Montgomery-Åsberg Depression Rating Scale). In the intent-to-treat analysis, 19 of 22 participants (86.4%) met remission criteria. Neuropsychological testing demonstrated no negative cognitive side effects.
- Conclusions: SAINT, an accelerated, high-dose, iTBS protocol with fcMRI-guided targeting, was well tolerated and safe. Double-blinded sham-controlled trials are needed to confirm the remission rate observed in this initial study.
The Study in Context:
- Machine-learning study finds EEG brain signatures that predict response to antidepressant treatments
- Should Cognitive Behavioural Therapy (not antidepressant drugs) be the first-line treatment for depression?
- Neuroengineering meets neuroethics to address treatment-resistant depression
- CVS Health: Cognitive behavioral therapy (CBT) apps may help you more than sleeping pills
- A call to action: We need the right incentives to guide ethical innovation in neurotech and healthcare
- New report: Empowering 8 Billion Minds via Ethical Development and Adoption of Neurotechnologies
- 10 highlights from the 2019 SharpBrains Virtual Summit


