Posts Tagged ‘Medicare’
Systematic review finds more clinical harm than benefits in Alzheimer’s “treatments” lecanemab, aducanumab, and donanemab
Study questions benefit of new Alzheimer’s drug (UGA Today): Last summer, the U.S. Food and Drug Administration fully approved the first drug shown to slow the progress of Alzheimer’s. But new research from the University of Georgia suggests that patients and caregivers may not experience any benefit from the drug in their daily lives. The drug, Leqembi,…
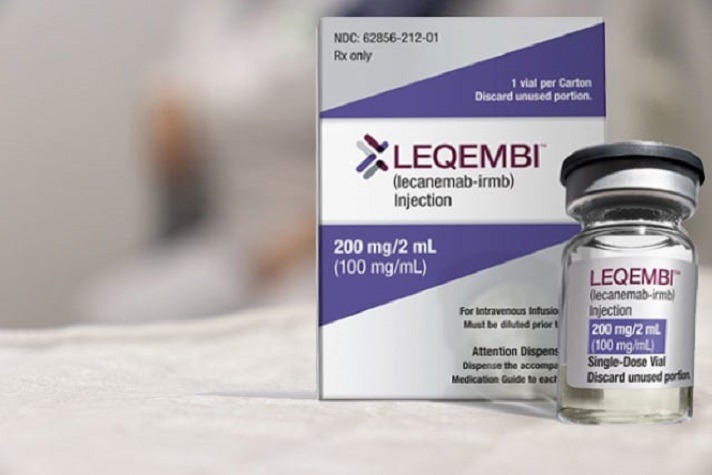
Read MoreHopes and Questions raised by Alzheimer’s drug Leqembi (lecanemab)
The FDA has approved Leqembi, the first disease-modifying treatment for early-stage Alzheimer’s and a precursor condition, mild cognitive impairment. Medicare has said it will pay for the therapy. Medical centers across the country are scrambling to finalize policies and procedures for providing the medication to patients, possibly by summer’s end or early autumn. It’s a…
Read MorePrice tag for a questionable Alzheimer’s treatment: $109,000 per patient, per year. Unclear yet: For how many years?
The real costs of the new Alzheimer’s drug, Leqembi — and why taxpayers will foot much of the bill (CBS News): The first drug purporting to slow the advance of Alzheimer’s disease is likely to cost the U.S. health care system billions annually even as it remains out of reach for many of the lower-income seniors…
Read MoreGrowing controversy over role of FDA and Medicare in promoting anti-amyloid drugs given limited benefit, high cost, severe side-effects
The War Over Whether Medicare Should Pay For The New Anti-Alzheimer’s Drugs (Forbes): The powerful Alzheimer’s Disease lobby is fighting a multi-billion-dollar battle on two fronts. It is quietly trying to limit restrictions the Food and Drug Administration puts on the use of new drugs aimed at slowing the progression of the brain disease. And…
Read MoreCMS: anti-amyloid drug Leqembi (lecanemab) doesn’t meet the “reasonable and necessary” standard required for wider Medicare coverage
CMS Sticks to Sharply Limited Coverage of New Alzheimer’s Drug, Leqembi (Managed Healthcare Executive): For now, CMS (Note: Centers for Medicare & Medicaid Services) is sticking to the coverage decision it made for Aduhelm (aducanumab) and applying it Leqembi (lecanemab). The decision limits Medicare coverage of the two Alzheimer disease’s drugs to Medicare beneficiaries who…
Read MoreUpdate on the aducanumab (Aduhelm) saga, retirement, financial advice, cognitive health, excessive worrying, neurotech, and more
Welcome to a new edition of SharpBrains’ e‑newsletter, providing this time a summary of the saga around the FDA approval of aducanumab (Aduhelm) as a supposed treatment for Alzheimer’s Disease, plus a range of timely research findings and resources for lifelong brain health. First, below are some key reads to navigate “probably the worst drug approval…
Read More