Should doctors prescribe lecanemab (Leqembi) to women? The answer, given available evidence, is probably No
 Data from the CLARITY trial earlier this year was supposed to be the crowning glory of the amyloid hypothesis, vindication for proponents of this long-held but much-maligned theory of Alzheimer’s disease.
Data from the CLARITY trial earlier this year was supposed to be the crowning glory of the amyloid hypothesis, vindication for proponents of this long-held but much-maligned theory of Alzheimer’s disease.
Yet the results left many feeling underwhelmed, and even the study authors noncommittal.
The CLARITY trial has many admirable features. It recruited close to 1800 people from around the world, pretty balanced between women and men. While the majority were white, 17% of the cohort was Asian and 12% Latino.
Choice of primary and secondary outcomes were impeccable. The primary outcome was the Clinical Dementia Rating sum of boxes (CDR-SB), a scorecard of sorts rated by a clinician across the domains of memory, orientation, problem solving, community affairs, home duties and personal care. Secondary outcomes included standard measures of global cognition, daily function, as well as biomarkers in the brain, from the CSF and blood.
So what happened? In short, the group who received fortnightly infusion of amyloid antibodies deteriorated by 1.21 points on the CDR-SB whilst the placebo group deteriorated by 1.66 points, the average difference being 0.45 points. That’s right, less than half a point on the CDR-SB! Less than the smallest change practically possible at an individual level and only calculable by comparing group averages.
At the same time, participants’ PET scans showed massive reductions of amyloid. Almost 75% of baseline cerebral fibrillar amyloid was removed; the majority of individuals moving from amyloid “positive” to amyloid “negative”. Whilst amyloid removal has been seen in prior trials using different anti-amyloids, this is the most convincing biological result yet and indeed difficult to imagine a better brain imaging result.
Having said this, the most troubling aspect was no statistically significant result–let alone clinically meaningful outcome–in women on the primary outcome or any clinical secondary outcome (Supplementary Figures 1–4). In other words, the headline readout was driven by positive outcomes in men, and it is beyond remarkable this was not discussed in the paper. This cannot be explained by a lack of statistical power, and suggests a more fundamental biological interaction at the level of therapeutic potency or mechanism of action. This will need a lot of careful analysis and further investigation to sort out.
Recall these ‘meh’ results are in the face of not inconsiderable or inconsequential risk of side effects. 26% of those receiving their fortnightly IV dose experienced an infusion reaction, and a 21.5% incidence of ARIA, a form of brain imaging abnormality related to bleeding and oedema unique to those treated by anti-amyloids. Whilst most turn out to be harmless, about a quarter are clinically significant, including stroke, and in any case ARIA abnormalities need expert diagnosis and management.
Altogether, lecanemab is the most potent anti-amyloid to date but like all its therapeutic cousins has not been able to show a clinically important difference compared to placebo, especially in women.
In a Letter to the Editor of the New England Journal of Medicine published a few days ago, myself and Professor Alvaro Pascual-Leone of Harvard communicate these concerns to the academic community. Our fundamental point is simple. There appears to be a repeating pattern in the trial data, whereby clinical outcomes are statistically significant in men but not so in women.
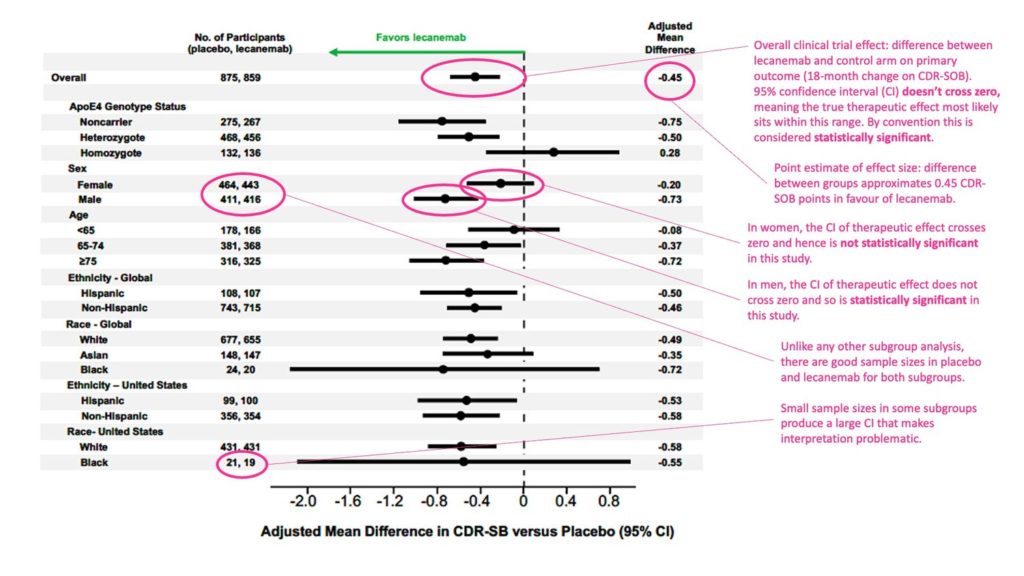
Below is the original paper’s data (Figure S1) for the primary end point of the trial, the CDR-SB with my annotation in pink. Click to expand the image:
For those unused to these forest plots, if the confidence interval of a particular comparison crosses zero then the difference between groups is considered statistically non-significant. Where the confidence interval does not cross zero then the comparison is significant.
Clearly, for CDR-SB the result was statistically significant in men (estimated difference between treatment vs placebo was 0.73 points). Equally clear is the result was non-significant in women (estimated mean difference 0.20 points).
On the basis of this trial, it is reasonable to conclude that the overall clinical impact of lecanemab as frequently cited (0.45 CDR-SOB point difference) arises from a real albeit weak therapeutic effect in men but negligible effect in women.
If lecanemab doesn’t work in women that is a big deal. Unfortunately, the response by the authors published alongside our Letter fails to shed any light on the matter.
Firstly, they state the trial was not powered to analyze individual subgroups. But subgroup analysis was pre-planned as per their published protocol, and hence the subgroup results merit consideration.
One explanation for the null result in women is a sampling error, but the size of the subgroups was healthy (n >400 for men and women) and the authors accept at face value that point estimates in women were lower than in men. If you’re going to interpret point estimates why not also consider their degree of precision, the confidence interval?
The alternative is that lecanemab is actually not effective in women. This rises in likelihood given null findings in women were also seen in all other clinical secondary endpoints in Figures S2-S4 (ADAS-COG14, ADCOMS, ACDS-MCI-ADL).
Secondly, they assert, “The subgroup analyses indicate that lecanemab performed better than placebo with respect to all clinical, biomarker, and quality-of-life outcomes among women, findings that were consistent with the overall efficacy.” They seem to be arguing that any numerical superiority in the treatment arm in women is sufficient, irrespective of the statistics. That is untenable and potentially dangerous.
To put it bluntly, if lecanemab doesn’t work in women it would be unethical to supply it to women.
Recall this costly immunotherapy comes with substantive risks, including high incidence of ARIA and even death. In biotech innovation such risk may be accepted in the context of a reasonable chance of clinical benefit, and should be avoided when those benefits are uncertain or unknown.
From a scientific perspective there are additional concerns. Why doesn’t it work in women? What is the biological basis for the decisive impact of sex on the mechanism of action? And is this specific to lecanemab or a problem for the whole class of anti-amyloids?
Engaging with these issues in an open and meaningful way is now critical for the credibility of this new drug, and I dare say, for the field at large.
 – Professor Michael Valenzuela is a Visiting Professor at the Centre for Healthy Brain Ageing at the University of New South Wales, Australia, member of the Clinical Consortium on Healthy Ageing of the World Health Organization and Co-Founder and CEO of Skin2Neuron Pty Ltd. This article is an edited combination of two previous blog posts by him at www.skin2neuron.org.
– Professor Michael Valenzuela is a Visiting Professor at the Centre for Healthy Brain Ageing at the University of New South Wales, Australia, member of the Clinical Consortium on Healthy Ageing of the World Health Organization and Co-Founder and CEO of Skin2Neuron Pty Ltd. This article is an edited combination of two previous blog posts by him at www.skin2neuron.org.