The European Society for Brain Stimulation opposes EU reclassification of TMS and tDCS, claiming a flawed safety assessment
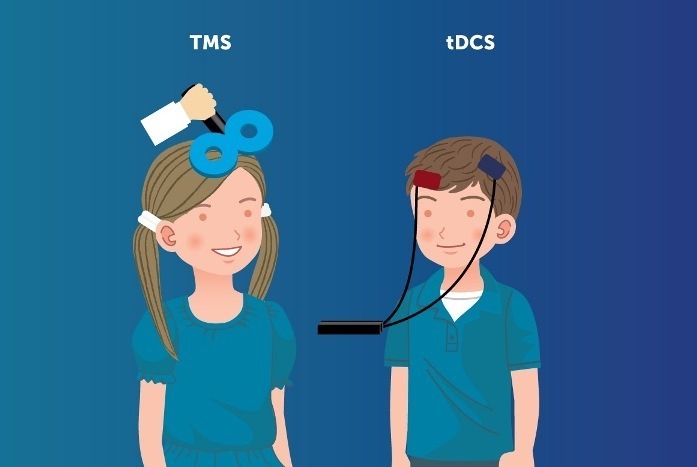 Manifesto: Opposition to EU Reclassification of TMS and tDCS equipment to Class III devices based on flawed evidence (European Society for Brain Stimulation):
Manifesto: Opposition to EU Reclassification of TMS and tDCS equipment to Class III devices based on flawed evidence (European Society for Brain Stimulation):
It has recently come to our attention that the EU has reclassified the NIBS equipments including rTMS and tDCS, as Class III devices, the category of highest risk, similar to invasive treatments, such as deep-brain stimulation implants. This reclassification has a major impact on our field, not only for manufacturers, but also for researchers, clinicians and patients and we judge this reclassification a mistake. First, the evidential basis for this change is a flawed assessment of the safety literature. Second, the consultation process has occurred without input from specialists or stakeholders professionally active in the field of NIBS. Neither the ESBS nor National Societies of Brain Stimulation nor individual experts within the European NIBS community were consulted on this change.
.. Although this new reclassification currently refers only to “products without an intended medical purpose”, the evidence adduced about the risks and adverse effects of rTMS and TES, — which serves as the justification for this reclassification‑, is gravely flawed. The EU has apparently assessed that NIBS poses a greater risk to patients’ safety than previously thought. This assessment is based on incorrect statements about rTMS and low intensity TES that are contradictory to the available scientific evidence, and many of the stated claims and assumptions are false (e.g., it is claimed that TMS/TES can induce “atypical brain development” or “abnormal patterns of brain activity”). Likewise, the prominent mentioning of rTMS/TES-related seizure risks contradicts the most recent consensus statement in the field based on actual clinical data which demonstrated that observed seizure rates are so much lower than previous guidelines advised, that the prior caution about seizure risk is no longer supported by scientific evidence (Rossi et al., 2020). To put this into perspective, the likelihood of a seizure from rTMS (0.003%) is lower than that associated with the use of antidepressants and antipsychotics (0.1–1, 5%), which are one of the most frequently prescribed treatments for depression (George et al., 2013). Moreover, when such seizures occur they do so mostly outside the clinician’s office, by contrast with rTMS (Perera et al., 2016). For low intensity tDCS, tACS, tRNS seizure risk is completely absent…
As an organisation and representatives of NIBS clinicians and professionals from across Europe, we, the ESBS, therefore disagree with this EU decision and in particular with the factually incorrect justifications of that decision to reclassify TMS and TES as invasive technologies that require medical class-III classification. The impact of this reclassification will result in higher costs, lower accessibility for patients to treatment and substantial delays in NIBS development for new clinical and research indications. … Therefore, if you are in agreement with the concerns stated above, we kindly ask you to protest this decision by sending an email to sante-med-dev@ec.europa.eu.
Upcoming webinar to discuss manifesto:
- January 27th, 11:30am US Eastern Time/ 5:30pm Europe CET time
- Registration required: HERE


