Neurostimulation device GammaSense by Cognito Therapeutics secures FDA Breakthrough Device Designation to explore Alzheimer’s Disease applications
Credit: The Picower Institute @ MIT
Cognito Therapeutics nets FDA breakthrough label for light, sound therapy for Alzheimer’s disease (Fierce Biotech):
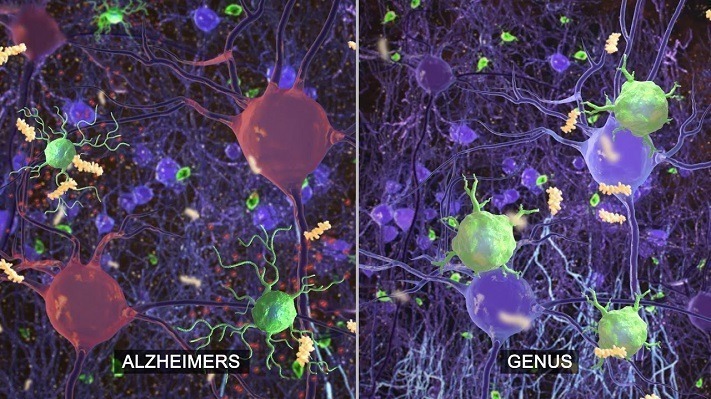
Using specific frequencies of flashing lights and sounds to stimulate the brain’s electrical activity, Cognito Therapeutics believes it can help treat Alzheimer’s disease by energizing neurons and reactivating the immune system.
Described as similar to a strobe light, but much faster, Cognito’s GammaSense stimulation system is built into a pair of opaque glasses worn with a set of headphones. The daily treatment has shown positive effects in mice, with changes in the brain’s hippocampus and auditory cortex including the reduction of amyloid plaques, according to a study published in Cell.
Now, with the treatment approach being studied in placebo-controlled human trials, the FDA said it has seen enough early clinical data to label the device as a potential breakthrough therapy—affording the company priority review and additional opportunities to work with agency officials through the project’s clinical and commercial development.
The Announcement:
Cognito Therapeutics Receives FDA Breakthrough Device Designation for Next-Generation Digital Therapeutic in Alzheimer’s Disease (press release):
Cognito Therapeutics, a clinical-stage company leading the development of a new class of disease-modifying digital therapeutics to treat neurodegenerative disorders, announced today its lead product has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for the treatment of cognitive and functional symptoms associated with Alzheimer’s disease. The product, a non-invasive neurostimulation device using gamma frequency technology, is the first in the Company’s pipeline of digital therapeutics for neurodegenerative diseases and other chronic indications…
“I am encouraged by Cognito’s innovative approach,” said Allan Levey, M.D., Ph.D., Professor and Chairman of the Department of Neurology at Emory University and Director of the Emory Goizueta Alzheimer’s Disease Research Center. “This strategy translating recent advances in non-invasive modulation of brain activity with sensory stimulation with light and sound has the potential to be an urgently needed safe, non-invasive, and effective treatment for millions of individuals with Alzheimer’s disease.”
The Study:
- Abstract: We previously reported that inducing gamma oscillations with a non-invasive light flicker (gamma entrainment using sensory stimulus or GENUS) impacted pathology in the visual cortex of Alzheimer’s disease mouse models. Here, we designed auditory tone stimulation that drove gamma frequency neural activity in auditory cortex (AC) and hippocampal CA1. Seven days of auditory GENUS improved spatial and recognition memory and reduced amyloid in AC and hippocampus of 5XFAD mice. Changes in activation responses were evident in microglia, astrocytes, and vasculature. Auditory GENUS also reduced phosphorylated tau in the P301S tauopathy model. Furthermore, combined auditory and visual GENUS, but not either alone, produced microglial-clustering responses, and decreased amyloid in medial prefrontal cortex. Whole brain analysis using SHIELD revealed widespread reduction of amyloid plaques throughout neocortex after multi-sensory GENUS. Thus, GENUS can be achieved through multiple sensory modalities with wide-ranging effects across multiple brain areas to improve cognitive function.
News in Context:
- Neuromodulation device Relivion gets FDA clearance to help patients with major depression who don’t benefit from antidepressant medications
- New report: Empowering 8 Billion Minds via Ethical Development and Adoption of Neurotechnologies
- We need to rethink neuroscience. And you can help us
- Five reasons the future of brain enhancement is digital, pervasive and (hopefully) bright


