Posts Tagged ‘Typing Cadence’
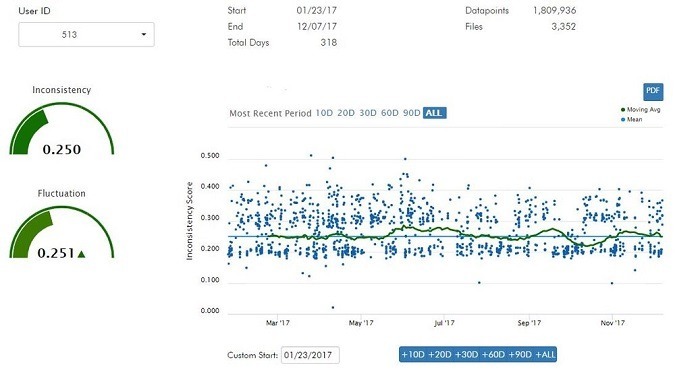
An idea gaining traction: Can measuring typing cadence help monitor and treat Parkinson’s Disease?
FDA grants breakthrough device designation to NeuraMetrix (press release): “The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation to the NeuraMetrix brain health monitoring solution. The designation is granted for devices “that provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions”. The approved use is monitoring…
Read MoreUpdate: Why monitoring Typing Cadence may help detect early Parkinson’s and Alzheimer’s Disease
_____ Time for SharpBrains’ first eNewsletter in 2018, offering a fascinating sneek peek into the rapidly growing toolkit to measure and improve brain health. (And don’t miss the fun teaser at the end!) New thinking: Brainnovations Winner Jan Samzelius on why monitoring Typing Cadence may help detect early Parkinson’s and Alzheimer’s Disease Firms Race to Find…
Read MoreJan Samzelius, CEO of Brainnovations Winner NeuraMetrix, encourages pioneers to focus on “simple, elegant solutions to big problems”
What surprised you the most from the Judges’ questions and feedback during the Brainnovations Pitch Contest last month? Even with only a brief presentation the judges immediately got the potential impact of our technology to measure and monitor brain health and began to think about other applications for us – several we had not thought about. That…
Read More