Posts Tagged ‘Major Depressive Disorder’
Precision psychiatry pioneer Alto Neuroscience raises $35M to advance digital biomarker-to-treatment platform
Alto Neuroscience Raises $35M Series B Financing (FinsMes): Alto Neuroscience, a Los Altos, CA-based neuro-tech company which specializes in precision psychiatry, raises $35M Series B Financing … The company intends to use the funds to advance lead candidates into Phase 2b studies in major depressive disorder … Proceeds from the financing will also be used…
Read MoreNeuromodulation device Relivion gets FDA clearance to help patients with major depression who don’t benefit from antidepressant medications
Neurolief wins FDA breakthrough nod for wearable neuromod for depression (Mass Device): Neurolief announced today that it received FDA breakthrough device designation for its Relivion DP system for treating major depression. Relivion is a wearable, non-invasive, multi-channel brain neuromodulation device designed as an adjunctive treatment to pharmaceutical management of major depressive disorder (MDD) in adults…
Read MoreOtsuka Pharma and Click Therapeutics enter partnership poised to transform mental healthcare
Otsuka, Click Therapeutics partner to create therapy for mental illness (Verdict Medical Devices): “Otsuka Pharmaceutical’s US division Otsuka America has partnered with Click Therapeutics to develop and commercialise a prescription digital therapeutic for treating major depressive disorder (MDD)
Read MoreTranscranial Direct Current Stimulation shows early promise to ameliorate depression, especially if combined with other therapies and dosage optimized
___ Transcranial Direct Current Stimulation Promising for Major Depressive Disorder (Psychiatry Advisor): “Transcranial direct current stimulation (tDCS) is an investigative modality for major depressive disorder (MDD) that has shown some promising results. Though it has a while before it is approved by the US Food and Drug Administration, clinicians and patients have been clamoring for…
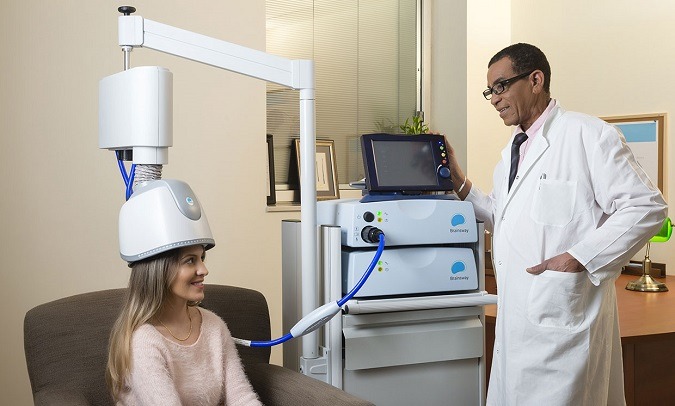
Read MoreFDA clears deep transcranial magnetic stimulation device to treat obsessive-compulsive disorder
BrainsWay’s Brain Stimulation Device Receives FDA Approval to Treat Obsessive-Compulsive Disorder (IEEE Spectrum): “In 2013, Jerusalem-based BrainsWay began marketing a new type of brain stimulation device that uses magnetic pulses to treat major depressive disorder. Now, thanks to positive results in a study of 100 patients, the company has received approval from the U.S. Food…
Read MoreSystematic evidence review finds cognitive behavioral therapy as effective as antidepressant medicines in treating depression
Depression: Treatment Beyond Medication (The Dana Foundation): “Major depressive disorder affects nearly 7 percent of people in the US aged 18 and up, according to the Depression and Bipolar Support Alliance. Those who seek treatment will most likely be prescribed a second-generation antidepressant medication such as a selective serotonin reuptake inhibitor (SSRI), tricyclic antidepressant (TCA),…
Read More