FDA clears deep transcranial magnetic stimulation device to treat obsessive-compulsive disorder
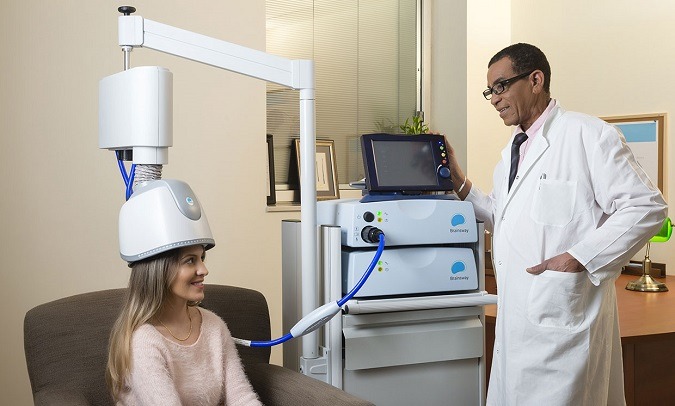
BrainsWay’s Brain Stimulation Device Receives FDA Approval to Treat Obsessive-Compulsive Disorder (IEEE Spectrum):
“In 2013, Jerusalem-based BrainsWay began marketing a new type of brain stimulation device that uses magnetic pulses to treat major depressive disorder.
Now, thanks to positive results in a study of 100 patients, the company has received approval from the U.S. Food and Drug Administration (FDA) to market the device for a second psychiatric condition—obsessive-compulsive disorder (OCD) …
Typically, noninvasive electrical and magnetic fields applied to the scalp, such as in transcranial direct current stimulation (tDCS), affect wide swaths on the brain, activating everything in their path. The most precise methods, deep brain stimulation techniques, require implanting an electrode deep into the brain.
The new device, however, relies on deep transcranial magnetic stimulation (Deep-TMS) to stimulate deep brain structures via powerful, carefully positioned electromagnets. The BrainsWay device stimulates the brain at greater depth and breadth than other TMS devices, claims company CEO Yaacov Michlin. “This allows us to noninvasively target previously unreachable areas of the brain,” he says.
The Study:
“BrainsWay’s FDA clearance for its OCD treatment was based on a pivotal, multicenter study demonstrating that after six weeks of treatment, there was a statistically significant improvement in the primary endpoint results for the active treatment group when compared to sham (p=0.0127) … The study was conducted in a total of 11 medical centers, with nine located in the United States, one in Canada and one in Israel. Patients who did not sufficiently respond to pharmacological or psychological treatment were enrolled in the trial and continued their pharmacological or psychological treatment during the study. The BrainsWay OCD treatment protocol consisted of twenty-minute sessions conducted five times per week, over the course of six weeks. Importantly, a tailored provocation was applied in each session to personalize the treatment for the patients’ specific disorder. Deep TMS treatment is non-invasive, requires no anesthesia and has been demonstrated to be safe and well-tolerated.”
More info in the press release
News in Context:
- FDA clears MindMaze GO neurorehabilitation platform, easing access to continued outpatient therapy
- FDA clears first CBT-based digital therapeutic to treat substance abuse disorders
- Study: Transcranial Direct Current Stimulation (tDCS) can reduce fatigue in patients with Multiple Sclerosis (MS)
- 10 Neurotechnologies About to Transform Brain Enhancement and Brain Health


