Posts Tagged ‘Food and Drug Administration’
AI-enabled chatbot Wysa receives FDA Breakthrough Device designation for patients with chronic pain, depression and anxiety
AI Behavior Health Chatbot App Fast-Tracked by FDA (Psychology Today): Recently the U.S. Food and Drug Administration (FDA) granted breakthrough device designation to Wysa’s AI-based digital mental health conversational agent that delivers cognitive behavioral therapy (CBT) via a smartphone to adults suffering from depression, anxiety, and chronic musculoskeletal pain
Read MoreDebate: Will digital therapeutics gain the required levels of awareness, adoption, reimbursement and fulfillment to become sustainable?
Can digital therapeutics become profitable? (MedTechDive): In 2020, the Food and Drug Administration cleared Akili Interactive’s video game to improve attention in kids with ADHD. It was the first time that a video game for treatment was cleared by the agency, and is one example of a digital therapeutic, a class of software-based treatments with…
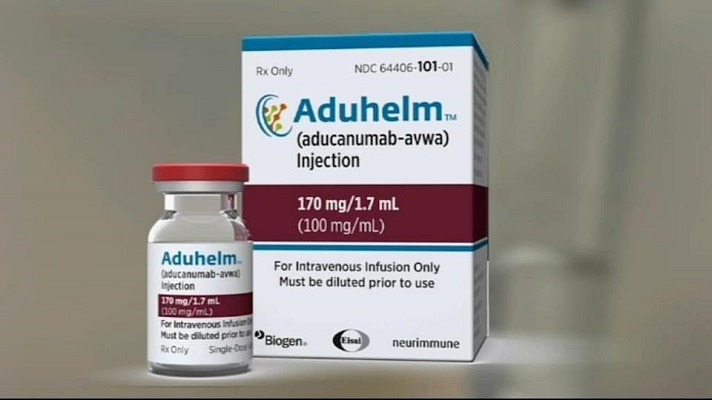
Read MoreSix guidelines to navigate the Aduhelm controversy and (hopefully) help patients with Mild Cognitive Impairment and early-stage Alzheimer’s Disease
The approval of a controversial new drug for Alzheimer’s disease, Aduhelm, is shining a spotlight on mild cognitive impairment — problems with memory, attention, language or other cognitive tasks that exceed changes expected with normal aging. After initially indicating that Aduhelm could be prescribed to anyone with dementia, the Food and Drug Administration now specifies that…
Read MoreThe explosion of mental health apps raises substantial opportunities–and also difficult questions
In the eyes of the tech industry, mental health treatment is an area ripe for disruption. In any given year, 1 in 5 adults in the U.S. experience a form of mental illness, according to federal estimates. And research indicates only about half of them receive treatment in a system that is understaffed and ill…
Read MorePrescription software firm Pear Therapeutics to go public via $1.6 billion SPAC deal, harnessing 3 FDA-authorized products and 14 candidates
Pear Therapeutics to Go Public in Roughly $1.6 Billion SPAC Deal (The Wall Street Journal): Medical technology company Pear Therapeutics Inc. has agreed to go public by merging with a blank-check company with ties to the Pritzker Vlock Family Office, betting on the growing role of prescription digital therapeutics. The proposed merger would give the…
Read MoreUS Senator Joe Manchin calls for a new FDA Commissioner to replace current (acting) one who “has repeatedly ignored public health concerns and shown a dereliction of duty” over opioids and aducanumab
Key Democrat Manchin Bashes FDA Leader on Alzheimer’s Approval (Bloomberg): Senator Joe Manchin, a moderate Democrat considered a crucial vote within the party’s slim Senate majority, said Janet Woodcock, the temporary head of the Food and Drug Administration, should be quickly replaced with a permanent leader. Manchin blasted an FDA decision to approve the controversial…
Read More