Posts Tagged ‘FDA’
Veterans Affairs won’t cover Biogen’s new “Alzheimer’s drug” given concerns over safety and lack of evidence
VA Health System Won’t Cover Biogen’s Alzheimer’s Drug (The Wall Street Journal): The Department of Veterans Affairs won’t cover Biogen Inc.’s new Alzheimer’s drug, the latest rebuke of the controversial treatment since it was approved earlier this summer. The VA decided not add the drug, called Aduhelm, to its formulary list of available medicines because…
Read MoreThe explosion of mental health apps raises substantial opportunities–and also difficult questions
In the eyes of the tech industry, mental health treatment is an area ripe for disruption. In any given year, 1 in 5 adults in the U.S. experience a form of mental illness, according to federal estimates. And research indicates only about half of them receive treatment in a system that is understaffed and ill…
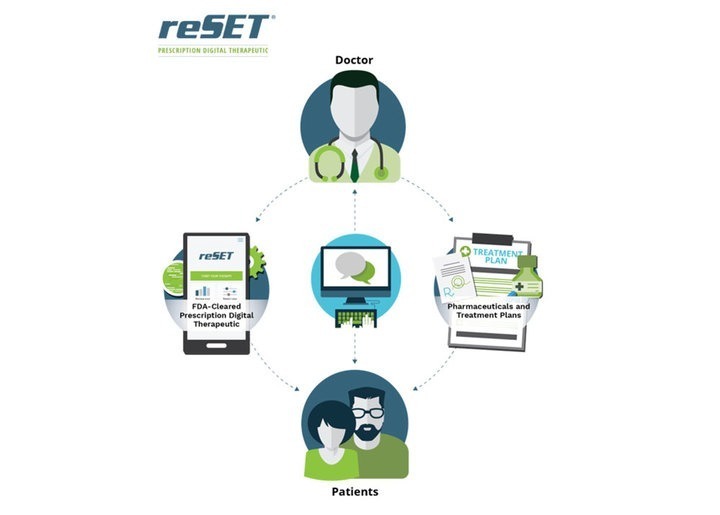
Read MorePrescription software firm Pear Therapeutics to go public via $1.6 billion SPAC deal, harnessing 3 FDA-authorized products and 14 candidates
Pear Therapeutics to Go Public in Roughly $1.6 Billion SPAC Deal (The Wall Street Journal): Medical technology company Pear Therapeutics Inc. has agreed to go public by merging with a blank-check company with ties to the Pritzker Vlock Family Office, betting on the growing role of prescription digital therapeutics. The proposed merger would give the…
Read MoreCan the controversial FDA approval of Aduhelm backfire and delay the discovery of actual Alzheimer’s treatments? (Yes, it can)
The U.S. Food and Drug Administration (FDA) recently approved aducanumab, the first treatment that aims to slow the progression of Alzheimer’s disease. But approval of the drug has provoked mixed reactions from the scientific community. Alzheimer’s disease is characterized by progressive memory loss, spatial disorientation and many other cognitive and behavioural disorders that ultimately lead…
Read MoreUS Senator Joe Manchin calls for a new FDA Commissioner to replace current (acting) one who “has repeatedly ignored public health concerns and shown a dereliction of duty” over opioids and aducanumab
Key Democrat Manchin Bashes FDA Leader on Alzheimer’s Approval (Bloomberg): Senator Joe Manchin, a moderate Democrat considered a crucial vote within the party’s slim Senate majority, said Janet Woodcock, the temporary head of the Food and Drug Administration, should be quickly replaced with a permanent leader. Manchin blasted an FDA decision to approve the controversial…
Read MoreFirst, do no harm? Six reasons to approach anti-amyloid drug Aduhelm cautiously, if at all
6 ways the FDA’s approval of Aduhelm does more harm than good (STAT News): Like many people, I was shocked when the Food and Drug Administration ignored the advice of its neurological drugs advisory panel and broadly approved Biogen’s new drug, Aduhelm, even for populations never included in the clinical trials to assess the drug.
Read More