Posts Tagged ‘breakthrough device’
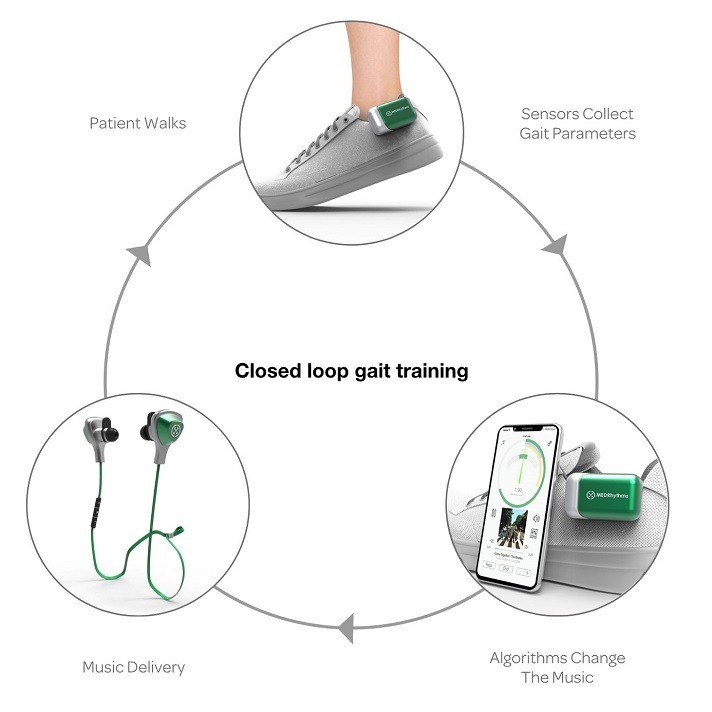
Integrating music, movement and stroke rehabilitation, MedRhythms raises $25M to develop and commercialize digital therapeutic
MedRhythms raises 25m to get patients back in tune after a stroke (TechCrunch): MedRhythms secured $25 million in Series B funding to advance its digital therapy platform aimed at measuring and improving someone’s ability to walk after they have experienced a neurologic injury or disease … Company co-founder and CEO Brian Harris was a neurologic music…
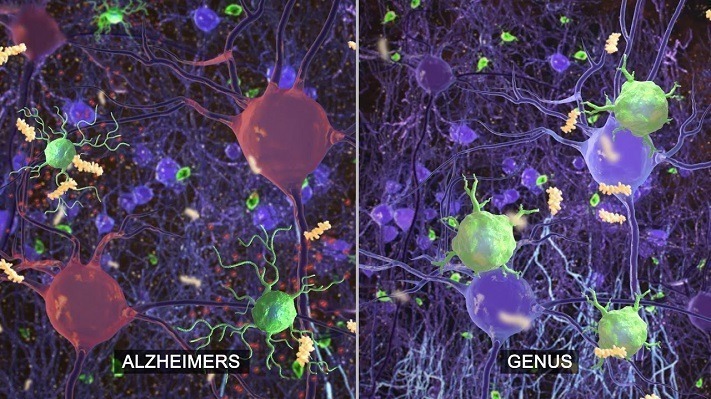
Read MoreNeurostimulation device GammaSense by Cognito Therapeutics secures FDA Breakthrough Device Designation to explore Alzheimer’s Disease applications
Cognito Therapeutics nets FDA breakthrough label for light, sound therapy for Alzheimer’s disease (Fierce Biotech): Using specific frequencies of flashing lights and sounds to stimulate the brain’s electrical activity, Cognito Therapeutics believes it can help treat Alzheimer’s disease by energizing neurons and reactivating the immune system.
Read MoreNeuromodulation device Relivion gets FDA clearance to help patients with major depression who don’t benefit from antidepressant medications
Neurolief wins FDA breakthrough nod for wearable neuromod for depression (Mass Device): Neurolief announced today that it received FDA breakthrough device designation for its Relivion DP system for treating major depression. Relivion is a wearable, non-invasive, multi-channel brain neuromodulation device designed as an adjunctive treatment to pharmaceutical management of major depressive disorder (MDD) in adults…
Read More