Posts Tagged ‘memory problems’
Six guidelines to navigate the Aduhelm controversy and (hopefully) help patients with Mild Cognitive Impairment and early-stage Alzheimer’s Disease
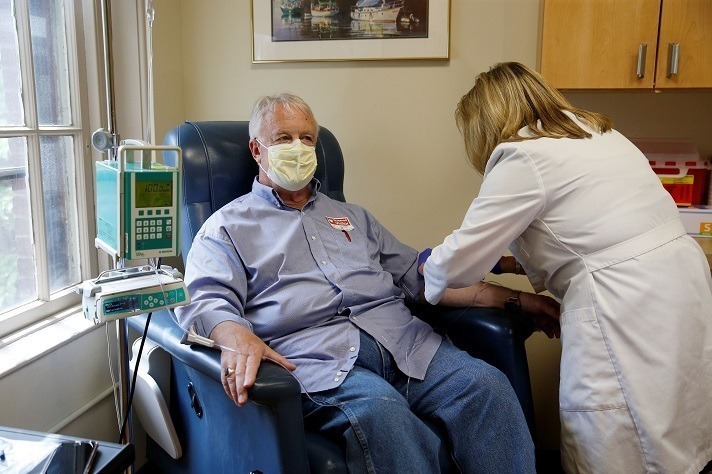
The approval of a controversial new drug for Alzheimer’s disease, Aduhelm, is shining a spotlight on mild cognitive impairment — problems with memory, attention, language or other cognitive tasks that exceed changes expected with normal aging. After initially indicating that Aduhelm could be prescribed to anyone with dementia, the Food and Drug Administration now specifies that…
Read More