Posts Tagged ‘EU’
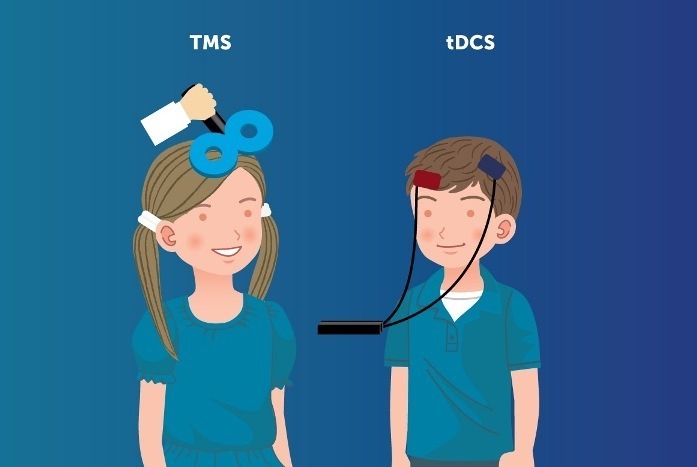
The European Society for Brain Stimulation opposes EU reclassification of TMS and tDCS, claiming a flawed safety assessment
Manifesto: Opposition to EU Reclassification of TMS and tDCS equipment to Class III devices based on flawed evidence (European Society for Brain Stimulation): It has recently come to our attention that the EU has reclassified the NIBS equipments including rTMS and tDCS, as Class III devices, the category of highest risk, similar to invasive treatments,…
Read MoreDebate: What are the ethics of discouraging much-needed innovation given potential privacy concerns?
Story description (CNN Money): Ned Sahin is founder and CEO of neurotechnology start-up Brain Power, whose tool “Empower Me” uses smart glasses like Google Glass to coach those with autism. It helps schoolchildren learn social and cognitive skills and can even guide adults through an interview process. Brain Power’s product is sold to many schools…
Read More