Posts Tagged ‘Biogen’
Update on the aducanumab (Aduhelm) saga, retirement, financial advice, cognitive health, excessive worrying, neurotech, and more
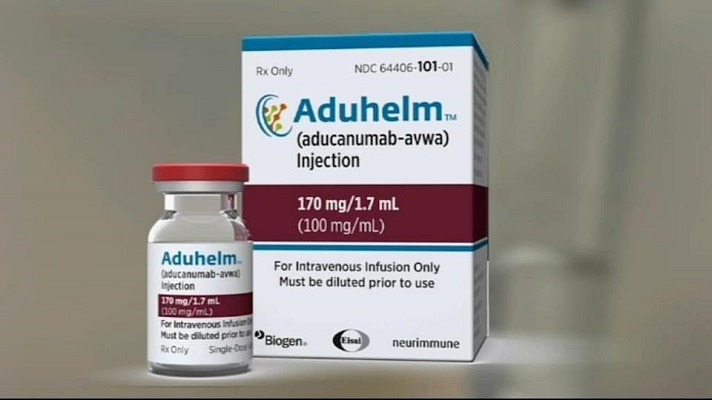
Welcome to a new edition of SharpBrains’ e‑newsletter, providing this time a summary of the saga around the FDA approval of aducanumab (Aduhelm) as a supposed treatment for Alzheimer’s Disease, plus a range of timely research findings and resources for lifelong brain health. First, below are some key reads to navigate “probably the worst drug approval…
Read MoreHealth payers–including Medicare and Point32Health–to question Aduhelm pricing and its “reasonable and necessary” use
State’s Second-Largest Health Insurer Slams Biogen For Costly Alzheimer’s Drug (The Boston Globe): The state’s second-biggest health insurer is threatening to limit or not cover Biogen’s new Alzheimer’s drug, accusing the Cambridge biotech of putting “excessive corporate profits” ahead of patients by charging $56,000 a year for the controversial treatment. Michael Sherman, chief medical officer…
Read MoreCan the controversial FDA approval of Aduhelm backfire and delay the discovery of actual Alzheimer’s treatments? (Yes, it can)
The U.S. Food and Drug Administration (FDA) recently approved aducanumab, the first treatment that aims to slow the progression of Alzheimer’s disease. But approval of the drug has provoked mixed reactions from the scientific community. Alzheimer’s disease is characterized by progressive memory loss, spatial disorientation and many other cognitive and behavioural disorders that ultimately lead…
Read MoreUS Senator Joe Manchin calls for a new FDA Commissioner to replace current (acting) one who “has repeatedly ignored public health concerns and shown a dereliction of duty” over opioids and aducanumab
Key Democrat Manchin Bashes FDA Leader on Alzheimer’s Approval (Bloomberg): Senator Joe Manchin, a moderate Democrat considered a crucial vote within the party’s slim Senate majority, said Janet Woodcock, the temporary head of the Food and Drug Administration, should be quickly replaced with a permanent leader. Manchin blasted an FDA decision to approve the controversial…
Read MoreGrowing backlash against the FDA approval of unproven Alzheimer’s treatment Aduhelm, by Biogen
ICER Issues Statement on the FDA’s Approval of Aducanumab for Alzheimer’s Disease (Institute for Clinical and Economic Review): The Institute for Clinical and Economic Review (ICER) believes that the FDA, in approving aducanumab (Aduhelm™, Biogen) for the treatment of Alzheimer’s disease, has failed in its responsibility to protect patients and families from unproven treatments with…
Read MoreThe new frontier in neurocognitive monitoring and dementia screening: the Apple Watch
Biogen to Launch Pioneering Study to Develop Digital Biomarkers of Cognitive Health Using Apple Watch and iPhone (press release): Biogen Inc. (Nasdaq: BIIB) today announced a new virtual research study, in collaboration with Apple, to investigate the role Apple Watch and iPhone could play in monitoring cognitive performance and screening for decline in cognitive health…
Read More