Posts Tagged ‘Alzheimers-disease’
Update on the aducanumab (Aduhelm) saga, retirement, financial advice, cognitive health, excessive worrying, neurotech, and more
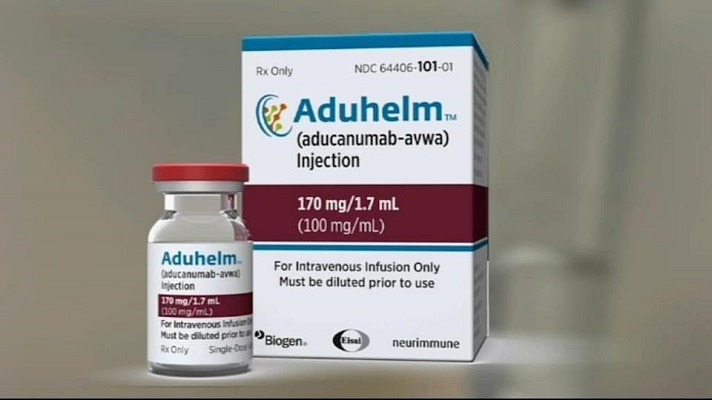
Welcome to a new edition of SharpBrains’ e‑newsletter, providing this time a summary of the saga around the FDA approval of aducanumab (Aduhelm) as a supposed treatment for Alzheimer’s Disease, plus a range of timely research findings and resources for lifelong brain health. First, below are some key reads to navigate “probably the worst drug approval…
Read MoreCan the controversial FDA approval of Aduhelm backfire and delay the discovery of actual Alzheimer’s treatments? (Yes, it can)
The U.S. Food and Drug Administration (FDA) recently approved aducanumab, the first treatment that aims to slow the progression of Alzheimer’s disease. But approval of the drug has provoked mixed reactions from the scientific community. Alzheimer’s disease is characterized by progressive memory loss, spatial disorientation and many other cognitive and behavioural disorders that ultimately lead…
Read MoreUS Senator Joe Manchin calls for a new FDA Commissioner to replace current (acting) one who “has repeatedly ignored public health concerns and shown a dereliction of duty” over opioids and aducanumab
Key Democrat Manchin Bashes FDA Leader on Alzheimer’s Approval (Bloomberg): Senator Joe Manchin, a moderate Democrat considered a crucial vote within the party’s slim Senate majority, said Janet Woodcock, the temporary head of the Food and Drug Administration, should be quickly replaced with a permanent leader. Manchin blasted an FDA decision to approve the controversial…
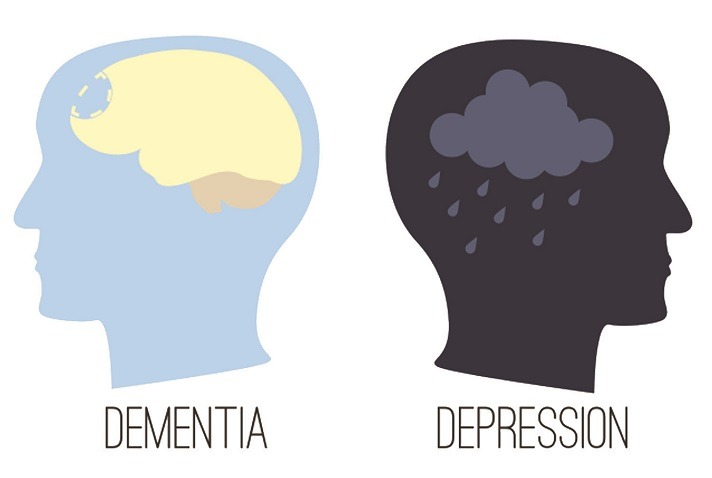
Read MoreDebate: Are depression and dementia two sides of the same coin? And, if they are, how to best approach treatment?
Every seven seconds, someone in the world is diagnosed with dementia. A typical case that I often see in my practice is as follows: A 76-year-old woman has a two-year history of progressive worsening of short-term memory and cognitive decline. She can’t recall the names of her grandchildren and is devastated by her deteriorating abilities.…
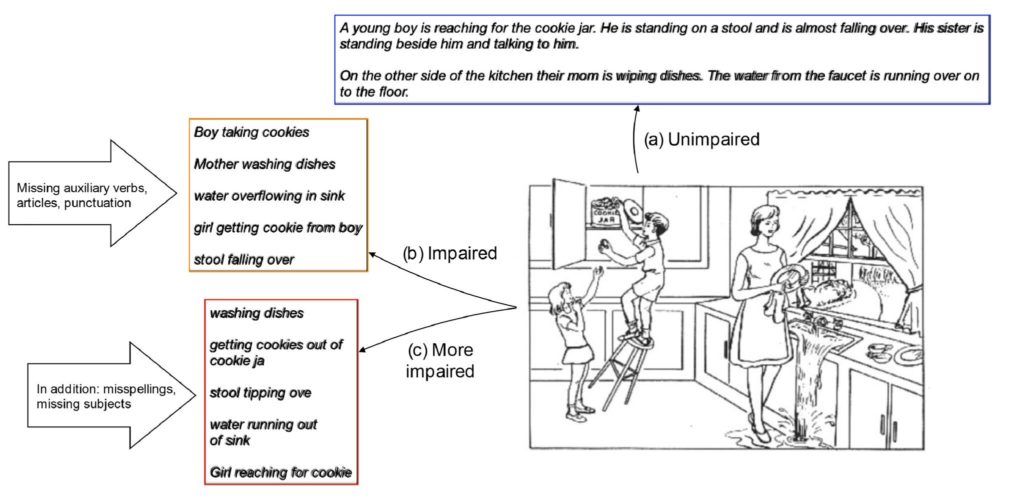
Read MoreStudy: Artificial intelligence program identifies linguistic markers that predict, with 70% accuracy, who gets Alzheimer’s Disease years later
Alzheimer’s Prediction May Be Found in Writing Tests (The New York Times): … the researchers looked at a group of 80 men and women in their 80s — half had Alzheimer’s and the others did not. But, seven and a half years earlier, all had been cognitively normal.
Read MoreStudy: Over-the-counter “brain enhancement” supplements in the US found both to a) contain multiple unapproved drugs and b) lack some ingredients listed on the label
Study: Your Brain Supplements Could Contain Dangerous, Illegal Ingredients (Being Patient): Brain supplements that claim to boost cognitive function are increasingly popular, growing from a $4 billion industry of about 4,000 unique products to a $40 billion industry with as many as 80,000 different products on the market.
Read More