Posts Tagged ‘Aduhelm’
Six guidelines to navigate the Aduhelm controversy and (hopefully) help patients with Mild Cognitive Impairment and early-stage Alzheimer’s Disease
The approval of a controversial new drug for Alzheimer’s disease, Aduhelm, is shining a spotlight on mild cognitive impairment — problems with memory, attention, language or other cognitive tasks that exceed changes expected with normal aging. After initially indicating that Aduhelm could be prescribed to anyone with dementia, the Food and Drug Administration now specifies that…
Read MoreWelcome to the Ultimate Neuroscience Lab: Your Smartphone
Welcome to a new edition of SharpBrains’ e‑newsletter, featuring this time six scientific reports and industry resources plus a fun illusion. #1. Top 10 Mental Health Innovations to Watch: Special SciAm/ WEF report Hoping you enjoy the great series over at Scientific American and especially #7, titled Welcome to the Ultimate Neuroscience Lab: Your Smartphone,…
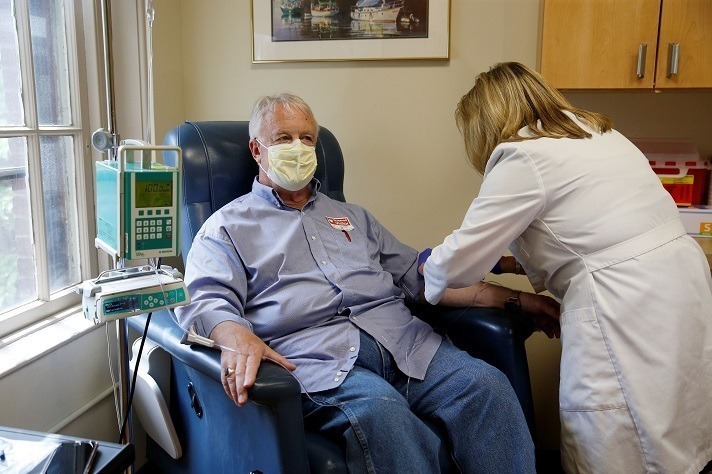
Read MoreStudy: Fewer than 1% of geriatric patients with cognitive complaints met Aduhelm research trial criteria. What can we expect about its real-world safety?
Biogen’s Aduhelm label far exceeds clinical trial population, study says. That could bring real-world surprises (Fierce Pharma): While the chatter surrounding Biogen’s controversial Alzheimer’s med Aduhelm has largely been centered on a pivotal Medicare reimbursement decision as of late, analysts are pointing to one new study that suggests there may be “room for surprises” when…
Read MoreVeterans Affairs won’t cover Biogen’s new “Alzheimer’s drug” given concerns over safety and lack of evidence
VA Health System Won’t Cover Biogen’s Alzheimer’s Drug (The Wall Street Journal): The Department of Veterans Affairs won’t cover Biogen Inc.’s new Alzheimer’s drug, the latest rebuke of the controversial treatment since it was approved earlier this summer. The VA decided not add the drug, called Aduhelm, to its formulary list of available medicines because…
Read MoreAlzheimer’s & Dementia researchers challenge FDA’s approval of Aduhelm given lack of evidence for beta-amyloid as a marker
Doctors Blast Biogen Alzheimer Approval as ‘Regulatory Failure’ (Bloomberg): Top researchers who advised the U.S. Food and Drug Administration on Biogen Inc.’s Alzheimer’s drug blasted the agency for approving it, calling the decision a “regulatory failure” that is “at odds with the evidence.”
Read MoreUpdate on the aducanumab (Aduhelm) saga, retirement, financial advice, cognitive health, excessive worrying, neurotech, and more
Welcome to a new edition of SharpBrains’ e‑newsletter, providing this time a summary of the saga around the FDA approval of aducanumab (Aduhelm) as a supposed treatment for Alzheimer’s Disease, plus a range of timely research findings and resources for lifelong brain health. First, below are some key reads to navigate “probably the worst drug approval…
Read More