Update on the aducanumab (Aduhelm) saga, retirement, financial advice, cognitive health, excessive worrying, neurotech, and more
 Welcome to a new edition of SharpBrains’ e‑newsletter, providing this time a summary of the saga around the FDA approval of aducanumab (Aduhelm) as a supposed treatment for Alzheimer’s Disease, plus a range of timely research findings and resources for lifelong brain health.
Welcome to a new edition of SharpBrains’ e‑newsletter, providing this time a summary of the saga around the FDA approval of aducanumab (Aduhelm) as a supposed treatment for Alzheimer’s Disease, plus a range of timely research findings and resources for lifelong brain health.
First, below are some key reads to navigate “probably the worst drug approval decision in recent U.S. history” — Dr. Aaron Kesselheim, the Professor of Medicine at Harvard Medical School who resigned rom the FDA Advisory Committee in protest.
#1. Growing backlash against the FDA approval of unproven Alzheimer’s treatment Aduhelm, by Biogen:
“The Institute for Clinical and Economic Review (ICER) believes that the FDA, in approving aducanumab (Aduhelm by Biogen) for the treatment of Alzheimer’s disease, has failed in its responsibility to protect patients and families from unproven treatments with known harms.”
#2. First, do no harm? Six reasons to approach anti-amyloid drug Aduhelm cautiously, if at all:
“The FDA’s approval of Aduhelm raises more questions and creates more problems than a new drug approval should. It’s time for governmental, professional, and advocacy entities to step in where Biogen and the FDA have failed and explain to patients, caregivers, and clinicians how this drug is not the “new day” in the fight against Alzheimer’s disease and needs to be approached cautiously, if at all.” — Dr. Sam Gandy, Professor of Neurology and Psychiatry at the Icahn School of Medicine at Mount Sinai, where he holds the Mount Sinai Chair in Alzheimer’s Research
“In short, while the amyloid hypothesis has faltered, the approval of aducanumab, which is based primarily on this theory, suggests that the theory may once again dominate research, and could reduce the chances of finding more promising treatments. For example, tau protein, which also accumulates in the brains of Alzheimer’s patients — long before the amyloid protein does — has been shown to be closely associated with the cognitive impairment resulting from the disease … we must not interrupt research on biomarkers and new therapeutic approaches.”
“I write today concerning the lack of permanent leadership at the Food and Drug Administration (FDA), and the continued tenure of Dr. Janet Woodcock as interim commissioner. Just last week, the FDA granted approval for Aduhelm (aducanumab), a treatment for Alzheimer’s, despite its advisory panel voting nearly unanimously against its approval, with no panel member voting in favor of approval”
(Let’s hope something useful emerges from this very unhealthy FDA decision. Quite disturbing, though, to notice the links between the opioid epidemic and the recent Aduhelm approval.)
“Under the broad label that FDA approved, the drug is available to all Alzheimer’s patients, and the agency did not place limits on treatment duration suggesting that patients could remain on the drug indefinitely. We are troubled by reports that those factors could lead the drug to command “somewhere between” the $37 billion we currently spend on Medicare Part B and the $90 billion we currently spend on Medicare Part D. This level of potential new spending, particularly for just one product with limited evidence of clinical efficacy thus far, tests the program’s resiliency.”
The stakes couldn’t be higher.
Now let’s review other important developments in June.
” … big do-it-yourself investing and trading venues like Vanguard Group, Fidelity Investments and Charles Schwab Corp. are strengthening some of the ways they detect possible signs of decline. Among other things, all three firms check for clients’ difficulty navigating security protocols or need for frequent password resets. In such cases, a designated family member might be informed.
Vanguard also checks client-call recordings for keywords—such as “confused” and “dementia”—that might signal trouble.”
“While retirement schemes like the 401(k) and similar programs in other countries are typically introduced to ensure the welfare of aging adults, our research suggests they need to be designed carefully to avoid unintended and significant adverse consequences. When people consider retirement, they should weigh the benefits with the significant downsides of a sudden lack of mental activity. A good way to ameliorate these effects is to stay engaged in social activities and continue to use your brains in the same way you did when you were working.
In short, we show that if you rest, you rust.”
 #8. The explosion of mental health apps raises substantial opportunities–and tough questions:
#8. The explosion of mental health apps raises substantial opportunities–and tough questions:
“Digital mental health can be viewed as a way to extend the mental resources that we have,” said David Mohr, who directs the Center for Behavioral Intervention Technologies at the Northwestern University Feinberg School of Medicine. A step-care model, for example, would allow patients with milder symptoms to be treated via technology while reserving in-person care for patients who need something more.
“Pear is one of nine companies invited to participate in the U.S. Food and Drug Administration’s (FDA) Precertification Pilot Program. Pear has developed and commercialized the first three FDA-authorized PDTs, has 14 product candidates, and is scaling its platform for third-party product distribution opportunities. The Company’s three FDA-authorized products, reSET®, reSET‑O® and Somryst®, address large market opportunities with more than 20 million patients suffering from substance and opioid use disorders and more than 30 million from chronic insomnia, in the U.S. alone, respectively.”
#10. Don’t worry, be happy: How excessive worrying may influence the rate of neurodegeneration:
“(Research findings) suggest that cognitive function may need to be monitored closely in individuals with affective disorders, as these individuals may be at particular risk of greater cognitive decline.”
#11. Smarter cars are coming soon … : Eye-tracking pioneer Smart Eye acquires MIT spin-off Affectiva to augment driver monitoring systems and more
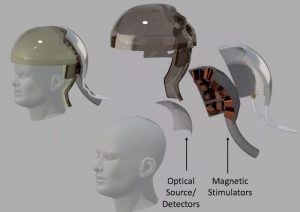 #12. And, much more: DARPA-funded nonsurgical neurotechnologies push the frontier of brain-machine interfaces
#12. And, much more: DARPA-funded nonsurgical neurotechnologies push the frontier of brain-machine interfaces
Finally, a quick cognitive exercise. Given the universal beauty of math, you don’t need to speak Spanish to try this quick teaser: Brain teasers en español: ¿cuál es el número que falta en el cuarto triángulo?
Wishing you a happy and healthy summer,
The SharpBrains Team

 #3.
#3.  #6.
#6. 
